Why Ice Is Slippery (and Scientists Still Can’t Fully Agree)
Why Is Ice So Slippery? (And Why Scientists Still Debate It)
By Peter Teoh, Science Writer
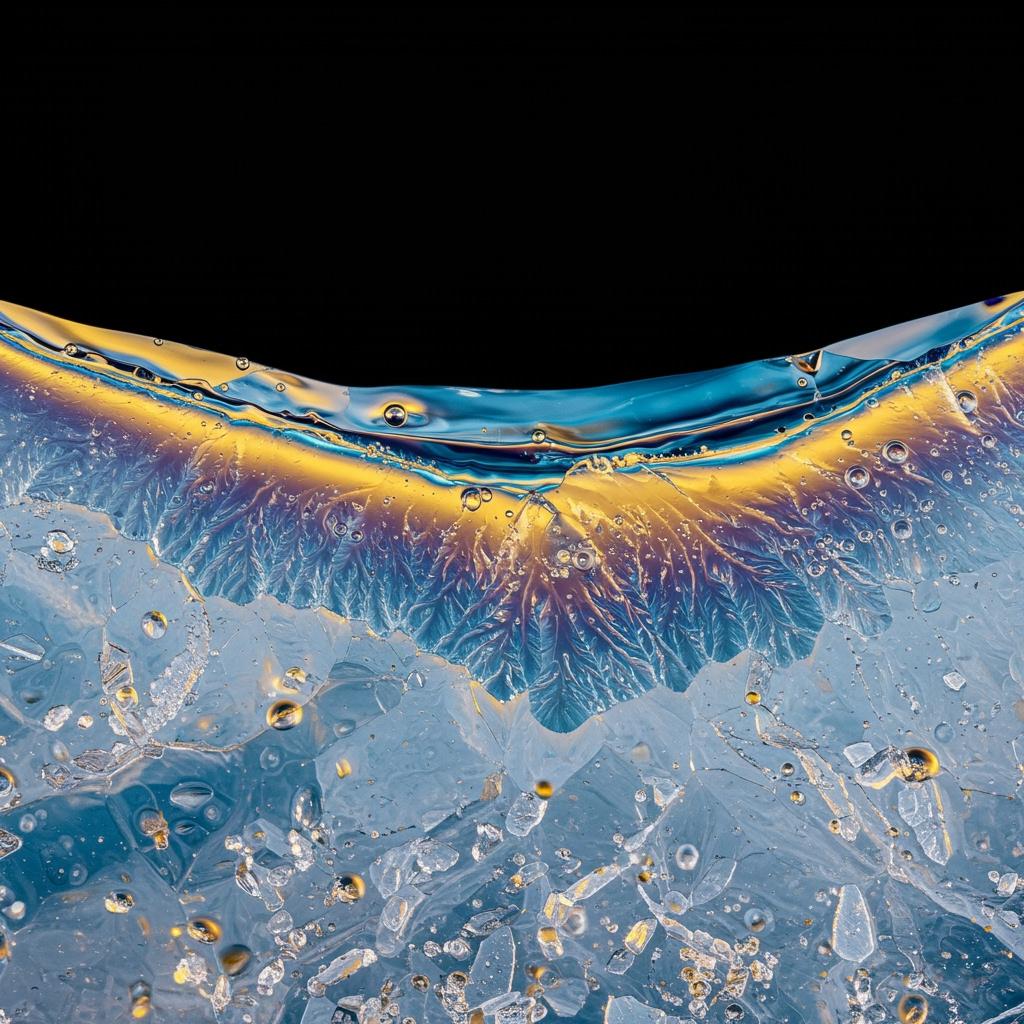 A microscopic view of ice’s surface reveals a thin, slippery layer of water—even below freezing—that helps explain why ice is so slick.
A microscopic view of ice’s surface reveals a thin, slippery layer of water—even below freezing—that helps explain why ice is so slick.
Have you ever wondered why ice is so slippery—why you can glide across a frozen pond but not a wooden floor? For over 200 years, scientists have scratched their heads over this everyday mystery. Even today, the answer isn’t as simple as you might think, and researchers are still uncovering new clues[3].
The Old Theory: Pressure Melts Ice
For nearly two centuries, the most popular explanation was that pressure from your foot or skate melts the ice, creating a thin layer of water that lets you slide[3]. But this idea has a big problem: ice is slippery even when you’re standing still, not just when you’re moving or pressing down hard[4]. If pressure were the whole story, you’d only slip when you stepped hard or skated fast—but that’s not what happens.
The Modern View: Pre-Melting and Surface Water
Today, most scientists agree that ice is slippery because of something called pre-melting. This is when the very top layer of ice becomes liquid-like even when the temperature is below freezing[1][4]. Think of it as a microscopic “skin” of water clinging to the ice, acting like a natural lubricant[5].
Recent studies using super-powerful microscopes have shown that the surface of ice isn’t perfectly solid. Instead, it’s a patchwork of different ice structures, with some areas more “loose” than others. As the temperature gets closer to freezing, this liquid-like layer gets thicker, making the ice even more slippery[1][2]. But when it’s extremely cold (think -40°C or below), this layer disappears, and ice feels more like sandpaper than a skating rink[3].
The Role of Friction
Friction—the rubbing force between your shoe and the ice—does play a part. When you slide, the heat from friction can melt a tiny bit more ice, adding to the slippery layer[3]. But friction isn’t the only cause, because the slippery layer exists even without movement. It’s a bit like oil on a car engine: it’s always there, making things slide more easily, but if you rub harder, it gets even slicker.
The Great Debate: What’s Really Happening?
Even with all this evidence, scientists still debate exactly how the slippery layer forms and how it behaves. Some think it’s all about the unique way water molecules arrange themselves at the surface. Others point to tiny defects and boundaries between different types of ice crystals that might help the slippery layer form[1][6].
What’s clear is that ice’s slipperiness is a delicate balance of temperature, surface structure, and a bit of physics magic that we’re still figuring out.
Fun Fact: You Can Walk on Ice Without Slipping
Believe it or not, there’s a technique called “ice walking” where you roll your feet onto the ice instead of sliding them. This reduces friction and makes it much harder to slip—proof that how you move matters, too[3].
Side Notes
- Ice Isn’t Always Slippery: At super-cold temperatures, ice loses its slippery layer and becomes rough and grippy[3].
- Not Just Water: Other solids can have slippery surfaces, but water’s unique properties make ice extra special.
- Skating Science: Ice skates work because their thin blades concentrate your weight, increasing pressure and helping to create that slippery layer—but remember, it’s not just the pressure doing the work!
Trending Sidebar
Did You Know?
- Scientists can now take pictures of ice’s surface with atomic-level detail, revealing the hidden world of water molecules in action[1].
- The debate over why ice is slippery goes back to the 1850s, with famous scientists like Michael Faraday weighing in[4].
- Next time you slip on ice, you’re experiencing one of nature’s coolest (and most mysterious) physics puzzles!
Reference Links
- Atomic-resolution imaging shows why ice is so slippery – Phys.org[1]
- Why Is Ice Slippery? New Study Overturns 200-Year-Old Physics Theory – SciTechDaily[3]
-
[Why Is Ice Slippery? Physics Today](https://pubs.aip.org/physicstoday/article/58/12/50/394684/Why-Is-Ice-Slippery-In-1859-Michael-Faraday) – AIP Publishing[4]
Leave a comment