The Molecules That Made Life Possible on Early Earth
How simple molecules sparked the origin of life
By Peter Teoh, Science Writer
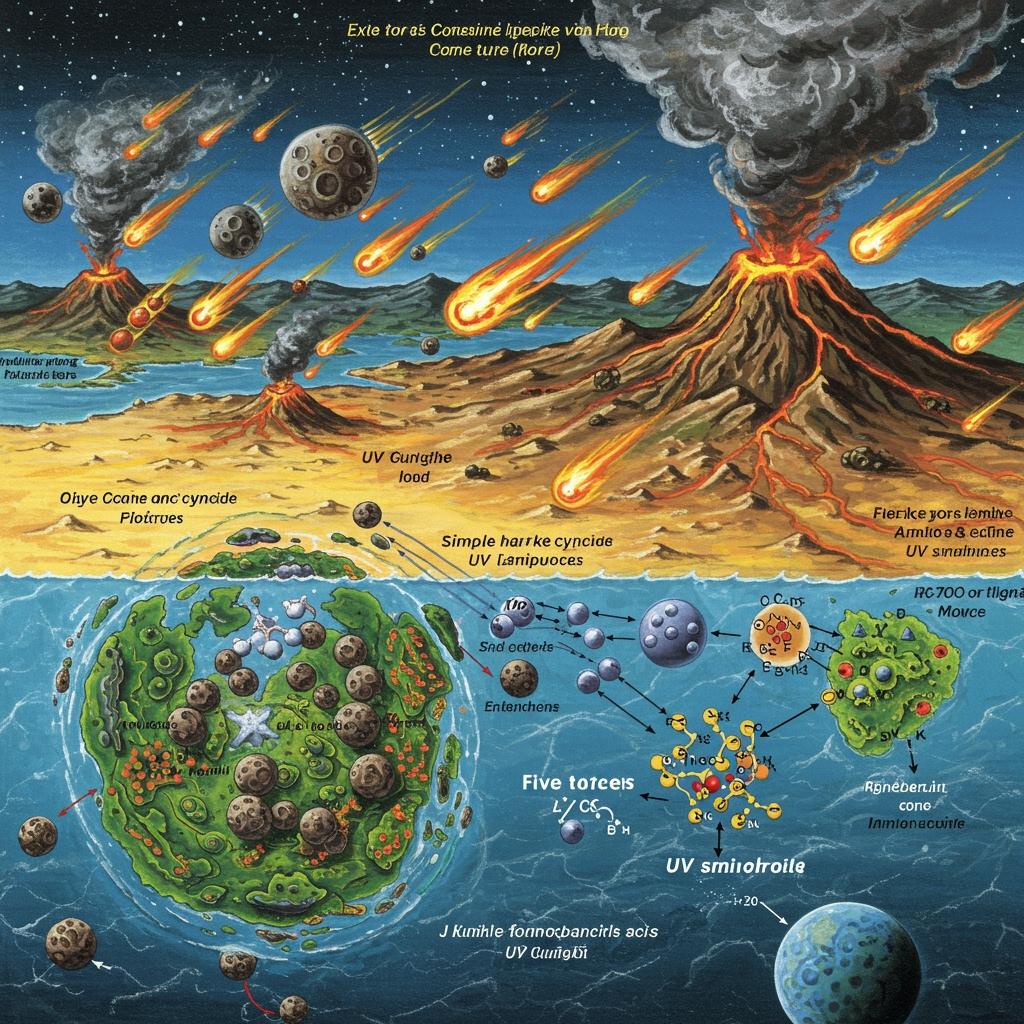 Early Earth was a dynamic place where simple molecules combined under the right conditions to create the building blocks of life.
Early Earth was a dynamic place where simple molecules combined under the right conditions to create the building blocks of life.
Imagine a world nearly 4 billion years ago, long before plants, animals, or even tiny microbes existed. The Earth was a harsh place—volcanoes erupted, comets crashed, and ultraviolet rays blasted its surface. Yet, in this chaotic environment, the first molecules that would eventually lead to life began to form. But what were these molecules, and how did they come together to make life possible? Let’s explore the fascinating story of the molecules that kickstarted life on early Earth.
What was early Earth like?
When Earth first formed, it was a hot, barren planet without oceans or an atmosphere like we know today. Scientists call this the Hadean eon. Over hundreds of millions of years, asteroid impacts brought water and gases that created oceans and an atmosphere. This created the perfect “chemical soup” where life’s ingredients could mix.
At this time, Earth’s surface was bombarded by ultraviolet (UV) light from the sun and energy from volcanic activity and meteor impacts. These energy sources were crucial—they powered chemical reactions that transformed simple inorganic molecules into the organic molecules essential for life.
The Building Blocks: What molecules mattered?
Life as we know it is built from a few key types of molecules:
- Amino acids: The tiny molecules that link together to form proteins, which do everything from building cells to speeding up chemical reactions.
- Nucleic acids (RNA and DNA): Molecules that carry genetic information.
- Lipids: Fat-like molecules that form cell membranes, creating the boundaries that protect cells.
- Sugars and simple carbohydrates: Provide energy and form the backbone of nucleic acids.
Scientists have found that these molecules could have formed naturally from simple gases like hydrogen cyanide (HCN), hydrogen sulfide (H₂S), carbon dioxide, and ammonia, all present on early Earth or delivered by comets. UV light and minerals acted as catalysts, helping these molecules combine in the right ways.
For example, a breakthrough study showed that hydrogen cyanide, hydrogen sulfide, and UV light could produce the precursors to nucleic acids, amino acids, and lipids all in one set of reactions. This means that many of life’s building blocks could have formed together in different environments and then mixed to create the first living systems.
How did these molecules come together?
One big puzzle is how simple molecules joined to form more complex molecules like peptides (short proteins) and RNA without enzymes (proteins that speed up reactions in living cells).
Recent research suggests sulfur-containing molecules might have played a key role, helping amino acids link into peptides on early Earth. These peptides could then fold into structures that performed useful functions, like catalyzing reactions.
Scientists also think that clay minerals or hydrothermal vents (hot underwater springs) provided surfaces where molecules could gather, react, and be protected from breaking down.
Why these molecules?
Interestingly, life today uses just 20 amino acids to build proteins, even though many more exist. Studies show these 20 are special because they link together efficiently and avoid unnecessary side reactions. This “selection” might have been a natural chemical filter billions of years ago.
Similarly, RNA is believed to be life’s first genetic molecule because it can both store information and catalyze reactions—a rare double talent.
The big picture: From molecules to life
The molecules didn’t just appear magically; they formed through a complex dance of chemistry powered by Earth’s unique environment. Over time, these molecules assembled into larger structures, eventually forming the first simple cells capable of replication and metabolism.
While many details remain a mystery, scientists continue to uncover clues by recreating early Earth conditions in labs and studying extreme environments on Earth today, like volcanic hot springs and deep-sea vents.
Discovering how life began here also helps us understand the possibility of life elsewhere in the universe, where similar chemical recipes might exist.
Side Notes
- RNA World Hypothesis: The idea that RNA molecules were the first to carry genetic information and catalyze reactions before DNA and proteins.
- Meteorite Delivery: Some organic molecules might have come from space, delivered by comets and meteorites, enriching early Earth’s chemistry.
- Energy Sources: UV light, volcanic heat, and lightning provided the energy needed to build complex molecules.
Trending Now
- Scientists simulate early Earth conditions to create new organic molecules in the lab.
- Discovery of amino acids in meteorites sparks debate on life’s cosmic origins.
- Advances in understanding peptide formation without enzymes open doors to synthetic biology.
References
-
Researchers may have solved origin-of-life conundrum, Science (2024) Science.org
-
The origin of life: The conditions that sparked life on Earth, Research Outreach (2023) ResearchOutreach.org
-
Origins of life: the molecules that could have unlocked peptide formation, Nature (2025) Nature.com
-
Hypotheses about the origins of life, Khan Academy KhanAcademy.org
-
A chemical clue to how life started on Earth, Scripps Research (2019) Scripps.edu
Leave a comment